
College
Department
Phone
Location
2008 - present: Associate Professor, Department of Chemistry, Stanislaus State, Turlock, CA
2006 - 2008: Lecturer, Department of Chemistry and Biochemistry, CSU Northridge, Northridge, CA
2004 - 2006: Postdoctoral Scholar, Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, CA
2004: Ph.D., University at Buffalo, The State University of New York, Buffalo, NY
1998-1999: Research Assistant, Procter & Gamble Pharmaceuticals, Norwich, NY
1998: B.S., State University of New York, College at Geneseo, Geneseo, NY
- "Green Catalytic Activation of H2O2 by Organometallic Compounds" Tanioka, R.; Ghali, M.; Drake, M. D. Poster presented at the College of Science 2013 Poster Celebration, Stanislaus State, Turlock, CA, May 2013. (pdf)
- "Synthesis of Fragrant Esters: Development of a New Organic Chemistry II Laboratory Experiment" Shabdin, A.; Drake, M. D. Poster presented at the College of Science 2013 Poster Celebration, Stanislaus State, Turlock, CA, May 2013.
- "Analysis of Conformational Influences Within (2R,3R)-Butanediol" Estrada, Z. M.; Drake, M. D. Poster presented at the College of Science 2013 Poster Celebration, Stanislaus State, Turlock, CA, May 2013. (pdf)
- "Green Catalytic Activation of H2O2 by Organometallic Compounds" Villines, K. M.; Tanioka, R.; Ghali, M.; Drake, M. D. Poster presented at the 25th Annual ACS Northern California Undergraduate Research Symposium, Santa Clara University, Santa Clara, CA, May 2013.
- "Synthesis of Fragrant Esters: Development of a New Organic Chemistry II Laboratory Experiment" Shabdin, A.; Drake, M. D. Poster presented at the 25th Annual ACS Northern California Undergraduate Research Symposium, Santa Clara University, Santa Clara, CA, May 2013.
- "Analysis of Conformational Influences Within (2R,3R)-Butanediol" Estrada, Z. M.; Drake, M. D. Poster presented at the 2013 Emerging Researchers National (ERN) Conference in STEM, Washington DC, March 2013.
- "Analysis of Conformational Influences Within (2R,3R)-Butanediol" Estrada, Z.; Drake, M. D. Poster presented at the College of Natural Sciences 2012 Poster Celebration, Stanislaus State, Turlock, CA, May 2012.
- "Green Catalytic Activation of H2O2 by Organometallic Compounds" Villines, K. M.; Lozano, E.; Drake, M. D. Poster presented at the College of Natural Sciences 2012 Poster Celebration, Stanislaus State, Turlock, CA, May 2012.
- "Analysis of Conformational Influences Within (2R,3R)-Butanediol" Estrada, Z.; Drake, M. D. Poster presented at the 33rd Annual Central California Research Symposium, CSU Fresno, Fresno, CA, April 2012.
- "Green Catalytic Activation of Hydrogen Peroxide by Organochalcogenide Compounds" Villines, K. M.; Lozano, E.; Drake, M. D. Poster presented at the 33rd Annual Central California Research Symposium, CSU Fresno, Fresno, CA, April 2012.
- "Analysis of Conformational Influences Within (2R,3R)-Butanediol" Estrada, Z.; Drake, M. D. Poster presented at the 2011 SACNAS National Conference, San Jose, CA, October 2011.
- "Homemade Chemical Instrumentation - Getting Creative When Budgets are Tight" Drake, M. D. Exhibit presented at the RSCA Week 2011 - Scholarship in the Spotlight research symposium, Stanislaus State, Turlock, CA, October 2011.
- "Analysis of Conformational Influences Within (2R,3R)-Butanediol" Estrada, Z.; Drake, M. D. Poster presented at the RSCA Week 2011 - Scholarship in the Spotlight research symposium, Stanislaus State, Turlock, CA, October 2011.
- "Conformational Analysis of Glycerol" Jauregui Caballero, R.; Drake, M. D. Poster presented at the 241st ACS National Meeting and Exposition, Anaheim, CA, March 2011. (pdf)
- "Conformational Analysis of Glycerol" Jauregui Caballero, R.; Drake, M. D. Poster presented at the RSCA Week 2010 - Passport to Scholarship research symposium, Stanislaus State, Turlock, CA, October 2010.
- "Conformational Analysis of Glycerol" Jauregui Caballero, R.; Drake, M. D. Poster presented at the 2010 SACNAS National Conference, Anaheim, CA, September 2010.
- Nkansah, R. A.; Liu, Y.; Alley, O. J.; Gerken, J. B.; Drake, M. D.; Roberts, J. D. J. Org. Chem. 2009, 74, 2344-2349.
- Smith, A. A.; Drake, M. D.; Roberts, J. D. J. Phys. Chem. A 2008, 112, 12367-12371.
- Detty, M. R.; Drake, M. D.; Tang, Y.; Bright, F. V. U.S. Patent 7 244 295, 2007.
- Drake, M. D.; Harsha, A. K.; Terterov, S.; Roberts, J. D. Magn. Reson. Chem. 2006, 44, 210-219.
- Rudner, M. S.; Jeremic, S.; Petterson, K. A.; Kent IV, D. R.; Brown, K. A.; Drake, M. D.; Goddard III, W. A.; Roberts, J. D. J. Phys. Chem. A 2005, 109, 9076-9082.
- Petterson, K. A.; Stein, R. S.; Drake, M. D.; Roberts, J. D. Magn. Reson. Chem. 2005, 43, 225-230.
- Detty, M. R.; Drake, M. D.; Tang, Y.; Bright, F. V. International Patent WO 2004/063292 A3, 2004.
- Drake, M. D. Ph.D. Thesis, University at Buffalo, The State University of New York, February 1, 2004.
- Drake, M. D.; Bright, F. V.; Detty, M. R. J. Am. Chem. Soc. 2003, 125, 12558-12566.
- Drake, M. D.; Bateman, M. A.; Detty, M. R. Organometallics 2003, 22, 4158-4162.
- Ahsan, K. A.; Drake, M. D.; Higgs, D. E.; Wojciechowski, A. L.; Tse, B. N.; Bateman, M. A.; You, Y.; Detty, M. R. Organometallics 2003, 22, 2883-2890.
- Francavilla, C.; Drake, M. D.; Bright, F. V.; Detty, M. R. J. Am. Chem. Soc.2001, 123, 57-67.
- Rosenberg, R. E.; Abel, R. L.; Drake, M. D.; Fox, D. J.; Ignatz, A. K.; Kwiat, D. M.; Schaal, K. M.; Virkler, P. R. J. Org. Chem. 2001, 66, 1694-1700.
- "An Improved Synthesis of Diorganoselenium and Tellurium Dendrimers" Drake, M. D.; Ahsan, K.; Detty, M. R. Poster presented at the 20th Annual Graduate Students Symposium, Buffalo, NY, May 2002.
- "Dendrimeric Catalysts for the Activation of Hydrogen Peroxide: Green Chemistry Oxidations" Drake, M. D.; Higgs, D. E.; Detty, M. R. Poster presented at the Sigma Xi Research Symposium, University at Buffalo, Buffalo, NY, April 2001.
- "Dendrimeric Catalysts for the Activation of Hydrogen Peroxide: Green Chemistry Oxidations" Drake, M. D.; Higgs, D. E.; Detty, M. R. Poster presented at the ACS Green Chemistry Symposium, Kodak Corporation, Rochester, NY, October 2000.
Join us Fridays from 2-3 p.m. in Naraghi 322 for the Chemistry department research meetings. All are welcome!

Members
- Keilah Villines
- Marina Ghali
- Nari Anowia
- Tamara Sada
- Zoila Estrada
- Anleel Shabdin
- Roger Tanioka
- Edwin Lozano
- Ninos Yacoub
- Rafael Jauregui
- Levi Merrell
- Ramson Mulhim
- Grant Langlois
- Joshua Casara
- Dongyang Chen
- Ani Julfayan
- Farbod Nader
- Yasaman Avyaee
- Truc Bui
- Emilia Janeke
- Cory Lagusker
- Danielle Lalimar
- Berj Varvaryan
Instrument Instructions
- 500 MHz NMR Instructions.pdf
- pH Meter Instructions.pdf
- Rotovap Procedure and Rules.pdf
- HP 5890 GC Instructions.pdf
Research Calculation Templates
Reference Tables
- Cambridge NMR Solvent Data.pdf
- NMR Shifts of Common Impurities.pdf
- 1H NMR Chemical Shifts.pdf
- 13C NMR Chemical Shifts.pdf
- IR Stretching Frequencies.pdf
- Properties of Common Organic Solvents.pdf
- Solvent Miscibility.pdf
Miscellaneous
Conformational Analysis
Structure leads to function. This is true, not only througout biology, but in chemistry as well. The macroscale function of a molecule is directly tied to the atomic scale structure that molecule adopts. The function of a complexly folded protein, or the physical properties of a simple water molecule are quick examples. For a molecule with intramolecular rotational freedom, there are an infinite number of structures, or conformations, it may adopt. Are there preferred conformations? If so, what are they? And what drives the molecule to adopt these conformations? These are the questions we try to answer.
To fully appreciate the purpose of this project, it is necessary that you first understand the chemist's visual perspective of molecules and molecular rotation. Below are two representations of meso-2,3-butanediol.

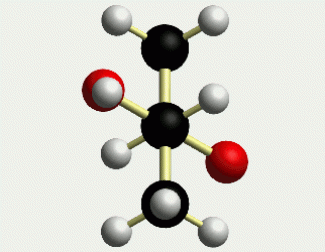
The first picture uses the wedge (coming out) and dash (going in) notation to present a three-dimensional perspective. The animation clearly shows the 3-D structure of the molecule. This molecule can adopt many conformations via rotation about each bond, but we are primarily concerned with rotation about the C2-C3 bond. This particular rotation is represented by each of the three images below.
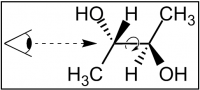
(showing your eye's perspective and the bond to rotate)
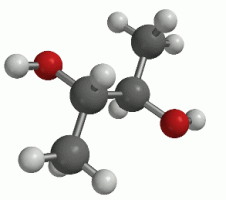
(a side-angled perspective of the rotation)
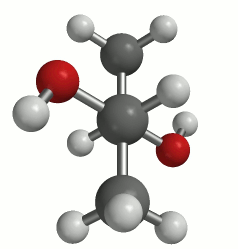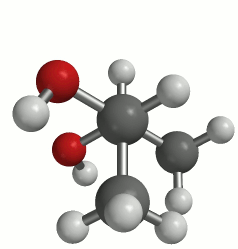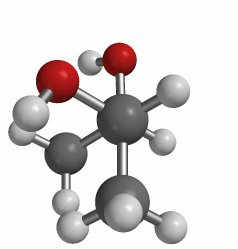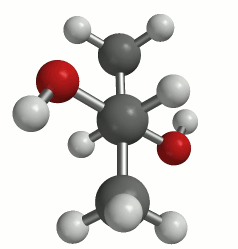
(a classic Newman projection perspective)
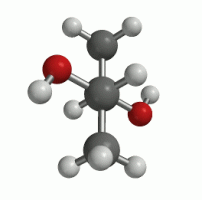
The previous animations are quite simple representations of intramolecular rotation. In reality, the molecule is twisting and bending, back and forth in all directions, with respect to each bond. The animation to the right presents a slightly more realistic picture of molecular rotation, although we are still focusing only on the C2-C3 bond. As the C2-C3 bond rotates, the molecule will experience conformations of high energy, and conformations of low energy. It is logical that the molecule will prefer to exist in conformations of low energy. How can we experimentally determine which conformations are low energy and preferred? The answer, as is often the case, is NMR!
A nuclear magnetic resonance (NMR) spectrum of our compound can give us conformational information. The coupling constant between the vicinal protons A and B is directly related to their dihedral angle, which is directly related to the rotational conformation of the molecule.

Acquire NMR spectrum
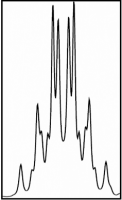
1H NMR spectrum of protons
A and B in CD3OD at 50 °C
Reproduce the NMR spectrum in a computer program to extract the coupling constant
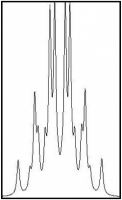
Computer simulated NMR spectrum
(JA-B = 5.1 Hz)
Results

In the absence of any conformational influence, each low energy rotamer shown to the left would comprise 33%. The results show the first rotamer is slightly favored. Why? For the typical Organic Chemistry student, the answer is the steric repulsion of the vicinal methyl groups. While that may be true in this case, there are many other possible explanations. Steric bulk is not the be-all and end-all. Other conformational influences may include:
- coulombic repulsion of the electronegative oxygen atoms and their lone pair electrons.
- potential intramolecular hydrogen bonding between the vicinal hydroxyl groups.
- potential intermolecular hydrogen bonding between the molecule and the solvent.
- the polarity of the conformer and the polarity of the solvent in which it is dissolved.
- hyperconjugation among vicinal sigma-bonding and sigma*-antibonding orbitals.
Why is any of this important? The conformational influences that dictate the structure of small molecules like that shown above, are the same influences which affect larger molecules like proteins. Small molecules are easier to analyze than proteins, and serve as nice model systems. If we can understand why a small molecule adopts the structure it does, we can understand better why a protein chain twists and folds the way it does. A deeper understanding of conformational structure provides chemists the information needed to choose a better solvent to achieve higher reaction yields, or perhaps build a better, more effective drug. The implications and applications of the knowledge gained from comformational analysis are broad and far-reaching across the scientific community.
Environmentally Friendly Oxidation Catalysts
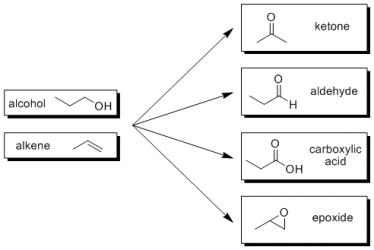
Oxidation reactions are an important part of organic chemistry and specifically organic synthesis. They are often one-step reactions resulting in the transformation of one functional group to another. Examples of common oxidation reactions are given to the right. Many different reagents can be used to execute these oxidative transformations, but the most commonly used reagents contain Cr6+. Examples of Cr(VI) oxidation reagents include dichromate in acid (Na2Cr2O7/H2SO4), PCC (pyridinium chlorochromate C5H5NH[CrO3Cl]), and PDC (pyridinium dichromate [C5H5NH]2[Cr2O7]). While these reagents work well for the intended oxidation (high yield, short reaction time), their primary disadvantage is their toxicity. Chromium(VI) is a heavy metal and must be handled and disposed accordingly. Furthermore, these reagents are stoichiometric, not catalytic. That means for every molecule we want to oxidize, you need at least one molecule of Cr(VI) reagent. This can get very hazardous and very expensive to dispose when oxidizing large quantities of material.
Hydrogen peroxide (H2O2) is another common oxidizing agent. When compared to Cr(VI) reagents, H2O2 suffers from long reaction times and low yields. But what H2O2 lacks in kinetic efficiency, it makes up for in environmental righteousness; over time, hydrogen peroxide degrades to water and oxygen! Wouldn't it be nice if we could make H2O2 work faster to combine its inherent "green" nature with the speed of Cr(VI) reagents? That is exactly our goal as we aim to synthesize catalysts to activate hydrogen peroxide.
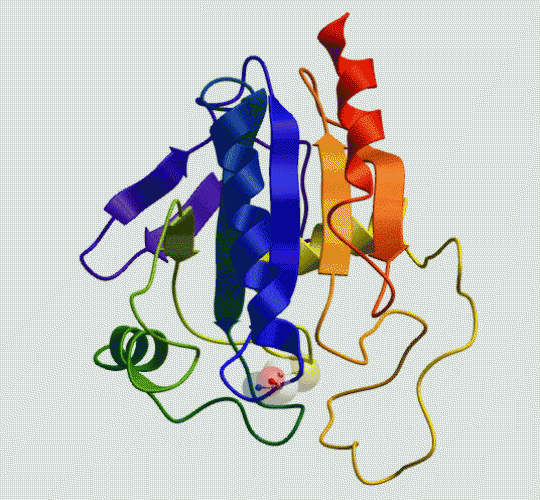
Mammalian gastrointestinal enzyme glutathione peroxidase 2, GPx2
(Molsoft rendering of pdb2he3)

L-cysteine
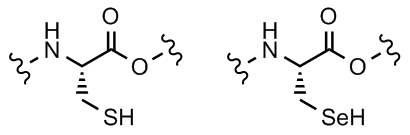
L-selenocysteine
Your body contains an antioxidant enzyme called glutathione peroxidase. Its purpose is to react with, and rid the body of peroxides. A signature peculiarity of glutathione peroxidase is the presence of the amino acid residue selenocysteine at the active site (highlighted near the bottom of the animation). In selenocysteine, the sulfur atom of a normal cysteine residue has been replaced with a selenium atom. The reactivity of selenium toward peroxides, and its role in the glutathione catalytic cycle serve as inspiration for our research.
In our research group we synthesize various organoselenium and organotellurium compounds. We then test their ability to catalytically activate H2O2 in the context of an oxidation reaction. Simple examples are diphenylselenide and diphenyltelluride. The proposed catalytic cycle for the activation of H2O2, by diphenylselenide, in the oxidation of Br- to "Br+" types of species (Br2, BrOH) is shown below. We are currently working toward the synthesis of durable, water soluble catalysts, and we are always seeking the fastest catalyst possible.

